Handling Flammable Gases in Industrial Areas
Read a summary using the INOMICS AI tool
Flammable gases, including natural gases like methane, pure substances such as hydrogen, and vapors from volatile liquids like acetone, require careful handling. Understanding their working limits is crucial for safety.
Always consult Safety Data Sheets for information on risks associated with these substances.
This article covers the properties of various flammable gases: Ammonia, Acetylene, Butane, Carbon Monoxide, Hydrogen, Methane, Propane, Ethane, Ethylene, Silane, and Chlorine Trifluoride.
Official Definitions of Gases and Vapors
By definition, according to the Globally Harmonized System of Classification and Labelling of Chemicals (GHS): A Flammable gas is a gas that has a flammable range at STP (Standard Temperature and Pressure) of 20‚ÑÉ and 101.3 kPa. These are considered normal conditions.
• "Gases" refers to materials that are in a gaseous state under normal atmospheric conditions. Examples of this would be hydrogen or methane.
• "Vapors" refers to the gases over a material that is a liquid under normal atmospheric conditions but emits gases within the flammable range under these atmospheric conditions.
Categories of Gases: Inert, Oxidizers, and Flammable
Gases fall into three main categories:
Oxidizers: These, like oxygen and bromine gas, aren't flammable but support combustion. Bromine, unique as a liquid non-metal, turns to gas only above 58℃, a temperature rarely reached on Earth.
Inert Gases: These gases, including noble gases like helium, argon, krypton, neon, and radon, as well as carbon dioxide, don't react with other substances.
Flammable Gases: Our primary focus, these gases can ignite under certain conditions.
Next, we'll delve deeper into flammable gases.
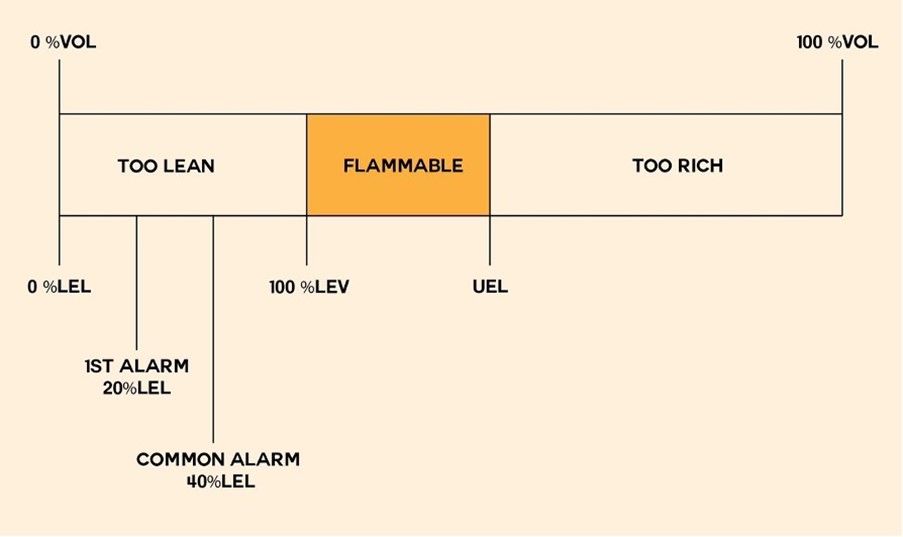
Understanding LEL & UEL in Gas Safety
When does a flammable gas ignite, and what are LEL & UEL? Flammable gases can cause explosions or fires when mixed with oxygen in the right proportions and exposed to an ignition source, like electric arcs from consumer electronics. This is why hazardous areas require special equipment, such as intrinsically safe cameras.
The Lower Explosion Limit (LEL) and Upper Explosion Limit (UEL) define the concentration range within which a gas can explode. For instance, hydrogen becomes explosive in air concentrations exceeding 4% but below 76%.
Exploring the Properties of Ammonia, Acetylene, and Butane
Ammonia (NH3)
Used in fertilizers and as a cost-effective, efficient refrigerant in large-scale operations like ice-skating rinks and food preservation. It's corrosive and toxic but easily detectable due to its strong odor, aiding in quick leak identification.
Acetylene (C2H2)
Highly explosive, acetylene is stored in acetone within cylinders containing a porous material to maintain stability. It's crucial to keep these cylinders upright to avoid safety issues.
Butane (C4H10) – Cooking Fuel
A safe, colorless, and abundant gas, butane is ideal for portable heating and cooking due to its blue flames and moderate energy yield. It doesn't easily vaporize in cold conditions. Iso-butane, used as a refrigerant, is structurally different but similarly flammable.

Hydrogen: Abundance and Environmental Impact
Hydrogen (H2) is the most abundant substance in the Universe, making up about 73% of all baryonic matter, with helium at 25%. All other elements, including the 116 on the Periodic Table, constitute the remaining 2%. This distribution reflects the vastness of stars like the Sun, primarily composed of hydrogen and helium, compared to smaller planets like Earth.
Hydrogen, powering the Sun's nuclear reactions, is not only abundant but also environmentally friendly when used as a fuel. It burns cleanly, producing only water and heat, and has no carbon footprint, except for production costs. This makes it a unique and valuable flammable gas.
Methane (CH4) – Natural Gas: Predominantly methane, natural gas also contains ethane, butane, pentane, propane, and helium. Odorless in its natural state, it's scented with substances like ethyl mercaptan for safety, giving it a 'rotten egg' smell to detect leaks.
Propane (C3H8): Known for its role in a major explosion during an illegal fuel transfer, propane is a versatile fuel for vehicles and rural power. It liquefies easily and remains gaseous even in low temperatures, unlike butane.
Ethane (C2H6): Separated from crude oil and used primarily in the plastic industry, ethane is also being explored for PVC production and as a cheaper alternative for acetic acid production. Additionally, it's used as a cryogenic coolant in electron microscopy, preventing water expansion and ensuring better sample quality. Some engines are now designed to switch between diesel and ethane fuel.
Silane (SiH4): Applications and Safety in Semiconductor Manufacturing
Silane, also known as Silicane (SiH4), is a pyrophoric and toxic gas used in semiconductor manufacturing for depositing pure silicon. Its pyrophoric nature, causing spontaneous ignition in air, demands meticulous handling. Innovatively, Japanese solar cell manufacturers have reduced its risks by blending it with hydrogen, resulting in not only safer handling but also more stable solar cells, showcasing the benefits of careful management of this hazardous material.
Understanding Explosive Gas Atmospheres: Real-World Examples
Explosive atmospheres require three elements: fuel, oxygen, and an ignition source, like sparking equipment or high temperatures. Let's explore some real-life scenarios:
Methane – Household Explosions: Methane, lighter than air, often doesn't accumulate at lower levels. However, it can build up underground, especially as a byproduct of bacterial activity in sewage systems, creating significant explosion risks.
Spray Painting Facilities – Industrial Explosions: These facilities must follow strict safety measures due to solvent use. Techniques like water curtains to trap overspray and electrically charged paint application are common.
Other Leakage Sources:
• Filling Drums: A critical point for leak detection.
• Piping System Flanges: Seal integrity is vital to prevent leaks.
• Sight Glasses in Equipment: These can be prone to leaks if not properly maintained.
In the petroleum industry, handling various flammable substances, enclosed operations are essential. This containment helps capture by-products for further processing or repurposing, ensuring safety and efficiency.
Conclusion: The Importance of Understanding Flammable Gases
Recognizing the characteristics and risks of flammable gases is key to preventing hazards. These gases, found in everyday items like nail polish remover and critical industrial fuels, are widely used in diverse applications.
Each flammable gas, from ammonia to butane, possesses unique properties and risks. Following Safety Data Sheets diligently is essential for risk mitigation. With informed caution, we can safely harness these gases in various settings, ensuring safety and well-being in both personal and professional spheres.
We would like to thank Armadex for their support in producing this article for New Engineer
Image Credits: Armadex
-
- PhD-Studiengang
- Posted 11 months ago
PhD Programme in Economics (with optional M.Sc. Economic Research)
Starts 1 Oct at University of Cologne and Universität zu Köln, WiSo-Fakultät and Cologne Graduate School in Management, Economics and Social Sciences in Köln, Deutschland -
- Konferenz
- Posted 3 weeks ago
44th RSEP International Conference on Economics, Finance and Business
Between 27 Nov and 28 Nov in Rome, Italien
-
- Konferenz
- Posted 4 days ago
45th RSEP International Multidisciplinary Conference
Between 4 Feb and 5 Feb in Lisbon, Portugal











